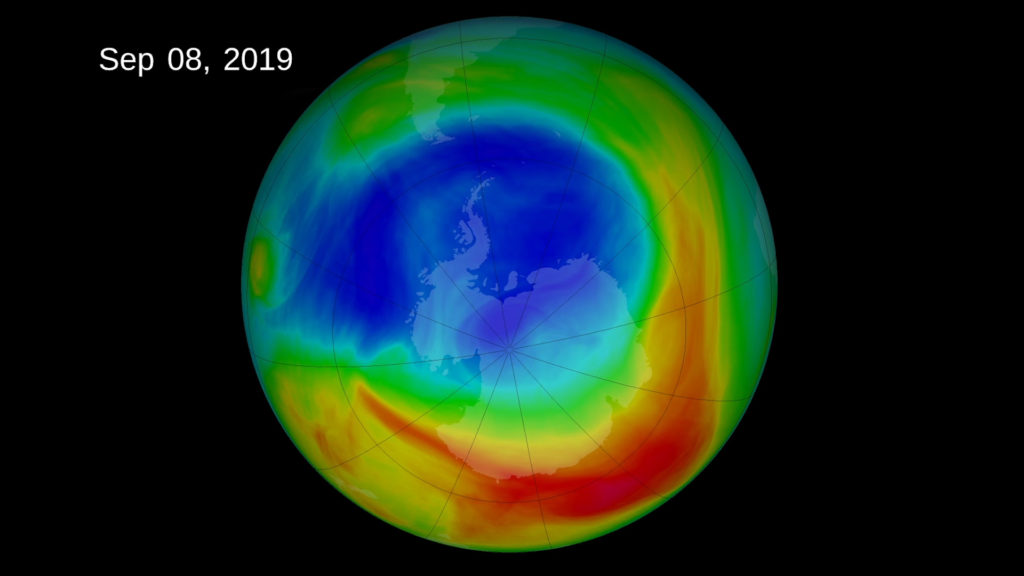
According to the NASA and NOAA study, abnormal weather patterns in the upper atmosphere over Antarctica dramatically limited ozone depletion in September and October, resulting in the smallest ozone hole observed since 1982.
This is the third time in the last 40 years that weather systems have caused warm temperatures that limit ozone depletion. Similar weather patterns in the Antarctic stratosphere in September 1988 and 2002 also produced atypically small ozone holes
The annual ozone hole reached its peak extent of 6.3 million square miles (16. 4 million square kilometers) on Sept. 8, and then shrank to less than 3.9 million square miles (10 million square kilometers) for the remainder of September and October, according to NASA and NOAA satellite measurements. During years with normal weather conditions, the ozone hole typically grows to a maximum area of about 8 million square miles in late September or early October.
However, Paul Newman, chief scientist for Earth Sciences at NASA’s Goddard Space Flight Center in Greenbelt, Maryland said, “it’s great news for ozone in the Southern Hemisphere, but it’s important to recognize that what we’re seeing this year is due to warmer stratospheric temperatures. It’s not a sign that atmospheric ozone is suddenly on a fast track to recovery.”
Ozone is a highly reactive molecule comprised of three oxygen atoms that occurs naturally in small amounts. Roughly seven to 25 miles above Earth’s surface, in a layer of the atmosphere called the stratosphere, the ozone layer is a sunscreen, shielding the planet from potentially harmful ultraviolet radiation that can cause skin cancer and cataracts, suppress immune systems and also damage plants.
According to the NASA, the Antarctic ozone hole forms during the Southern Hemisphere’s late winter as the returning Sun’s rays start ozone-depleting reactions. These reactions involve chemically active forms of chlorine and bromine derived from man-made compounds. The chemistry that leads to their formation involves chemical reactions that occur on the surfaces of cloud particles that form in cold stratospheric layers, leading ultimately to runaway reactions that destroy ozone molecules. In warmer temperatures fewer polar stratospheric clouds form and they don’t persist as long, limiting the ozone-depletion process.


